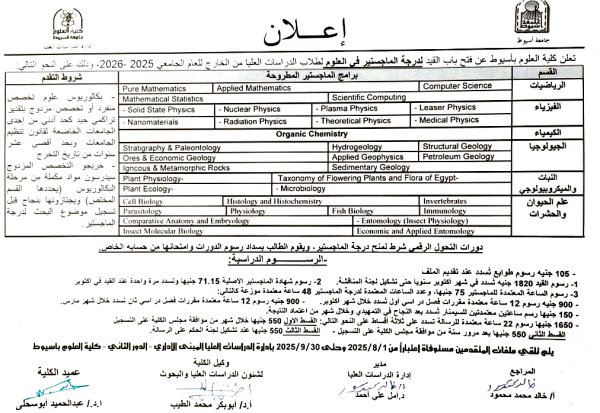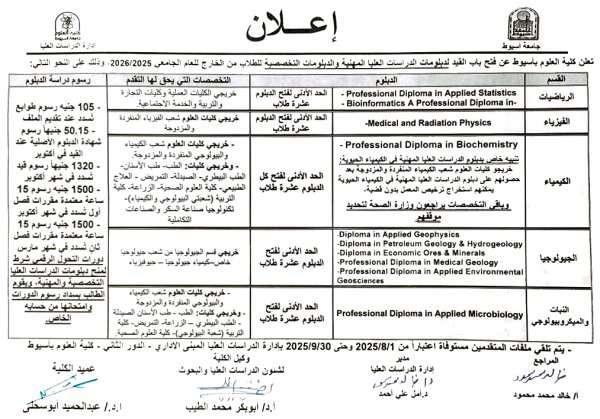

Announcement of the opening of registration for technical and specialized diplomas for the academic year 2025-2026
Invitation to the opening of the open botanical garden at Assiut University. Sunday, July 13, 2025, at 9:00 AM.
Insights into structural and spectral properties of cobalt II complexes bearing asymmetric thiourea ligands and their remarkable bioactivities against Gram negative bacteria
Research Authors
Hanan K Mosbah, Aref AM Aly, Amna SA Zidan, Ahmed BM Ibrahim, Jaromir Marek, Ghada Abd-Elmonsef Mahmoud
Research Date
Research Department
Research Journal
Inorganic Chemistry Communications
Research Member
Research Year
2025
Synthesis Characterization X-Ray Structure and Antifungal Activity of a Platinum II Thiourea Complex
Research Authors
Aref AM Aly, Hanan K Mosbah, Amna SA Zidan, Ahmed BM Ibrahim, S Mark Roe, Ghada Abd‐Elmonsef Mahmoud
Research Date
Research Department
Research Journal
ChemistrySelect
Research Member
Research Year
2025
Rational Design of a Copper I Complex Derived from an Asymmetric Thiourea Ligand with High Antifungal Activities
Research Authors
Hanan K Mosbah, Aref AM Aly, Amna SA Zidan, Ahmed BM Ibrahim, S Mark Roe, Ghada Abd‐Elmonsef Mahmoud
Research Date
Research Department
Research Journal
ChemistrySelect
Research Member
Research Year
2025
A newly developed cobalt(II) complex derived from a thiourea derivative and assessment of its potential bioapplicability against plant root pathogens
Research Authors
Hanan K Mosbah, Aref AM Aly, Amna SA Zidan, Ahmed BM Ibrahim, Jaromir Marek, Ghada Abd-Elmonsef Mahmoud
Research Date
Research Department
Research Journal
journal of Coordination Chemistry
Research Member
Research Year
2025
Insights into structural and spectral properties of cobalt(II) complexes bearing asymmetric thiourea ligands and their remarkable bioactivities against Gram negative bacteria
Research Authors
Hanan K. Mosbah, Aref A.M. Aly, Amna S.A. Zidan, S. Mark Roe, Ghada Abd-Elmonsef Mahmoud, Ahmed B.M. Ibrahim
Research Date
Research Department
Research Journal
Inorganic Chemistry Communications
Research Member
Research Pages
115143
Research Vol
181
Research Year
2025
Rational Design of aCopper(I) Complex Derived from an Asymmetric Thiourea Ligand with High Antifungal Activities
Research Authors
Hanan K. Mosbah, Aref A. M. Aly, Amna S. A. Zidan, Ahmed B. M. Ibrahim, S. Mark Roe, Ghada Abd-Elmonsef Mahmoud
Research Date
Research Department
Research Journal
ChemistrySelect
Research Member
Research Pages
e03254
Research Vol
10
Research Year
2025
Carbon gel materials: synthesis, structural design, and emerging applications in energy and environmental technologies
Research Authors
Md Shariful Islam, Shreyase Kundu, Mst Samsunnahar, Tasmina Khandaker, Ahmed B. M. Ibrahim, Md Al Amin Mia Anik, Md. Kamrul Hasan, Muhammad Sarwar Hossain
Research Date
Research Department
Research Journal
Materials Advances
Research Member
Research Pages
7153-7206
Research Vol
6
Research Year
2025