👇

 Do you have any questions? (088) 2080369 - 2345622 Pharmacy_QAAU@pharm.aun.edu.eg
Do you have any questions? (088) 2080369 - 2345622 Pharmacy_QAAU@pharm.aun.edu.eg
The semester work and practical exam grades for the course “Pharmacognosy -2” – for Second-Year Pharm-D students, First Semester of the Academic Year 2025/2026.
Surveys for Administrative Staff for the Academic Year 2024/2025

Survey for Staff at the Faculty Job Satisfaction
https://forms.gle/i4zdmyu6D2T8RyPc7
Survey for the Administrative Staff's Opinion on the Work Environment
https://forms.gle/VYC43tW2tENm17Ae7
Survey on the Technological Services Unit at the Faculty
https://forms.gle/GfNNtyK6EGm3KuS98
Survey on the Faculty of Pharmacy Website
Surveys for Faculty Members and Staff Assistant for the Academic Year 2025/2026

Survey for Faculty Staff and Staff Assistant Job Satisfaction
https://forms.gle/6r39t3goUVf7uQa39Survey for Faculty Staff and Staff Assistant Opinion on the Work Environment
https://forms.gle/rR67oiz8yFytcnod9Survey on the Technological Services Unit at the Faculty
https://forms.gle/GfNNtyK6EGm3KuS98
Survey on the Faculty of Pharmacy Website
Microwell-based spectrophotometric analysis of pemigatinib via formation of charge transfer complexes: Eco-friendly and high-throughput methods
Two prototype eco-friendly 96-microwell spectrophotometric methods with high throughput for the determination of pemigatinib (anticancer drug) in Pemazyre® tablets were developed. The two methods involved in microwell one-step formation of colored charge transfer complexes upon interaction of pemigatinib, as an electron donor, with two different benzoquinone electron acceptors: 2,5-dihydroxy-3,6-dichloro-1,4-benzoquinone (chloranilic acid) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. The molar ratios of charge transfer complexes, determined by Job’s method, were found to be 1:1. Computational density functional theory analysis of charge transfer complexes confirmed a π→π* single-electron transfer mechanism. An additional hydrogen bond in the chloranilic acid complex was established, further stabilizing the assembly. Conversely, in the 2,3-dichloro-5,6-dicyano-1,4-benzoquinone complex, only the π-stacking interaction is present, with the intramolecular NH···N hydrogen bond remaining intact. The interactions were performed on 96-well transparent plates, and the absorbances of the charge transfer complexes were measured at 530 and 465 nm for pemigatinib–chloranilic acid and pemigatinib–2,3-dichloro-5,6-dicyano-1,4-benzoquinone complexes, respectively. The microwell spectrophotometric method procedures were refined and validated following the International Council of Harmonisation (ICH) standards. The limits of detection of the methods were 8.5 and 4.1 µg well−1 for chloranilic acid and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone methods, respectively. These methods proved highly reliable for assessing pemigatinib in Pemazyre® tablets and ensuring uniformity. The eco-friendliness/greenness of the microwell spectrophotometric methods was confirmed by three distinct metrics. The methods’ one-step reactions and capacity for processing numerous samples simultaneously contribute to their high-throughput capabilities. In conclusion, this study represents the first two facile, green, and high-throughput microwell spectrophotometric methods for pemigatinib analysis for quality control purposes. In addition, it is the first work exploring the interactions of pemigatinib with chloranilic acid and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone.
The Educated University Student" Survey for University Student Registration for the 2025–2026 Academic Year
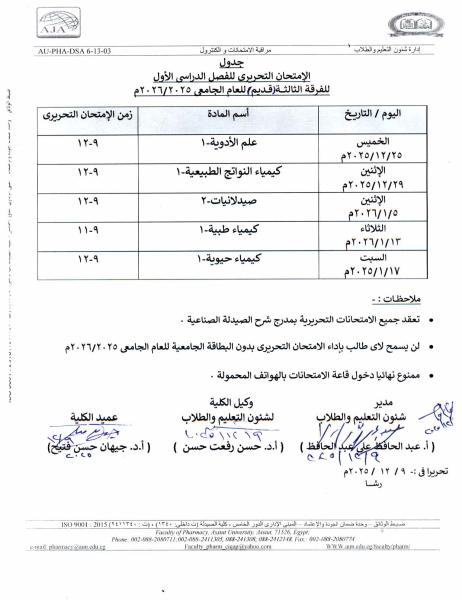
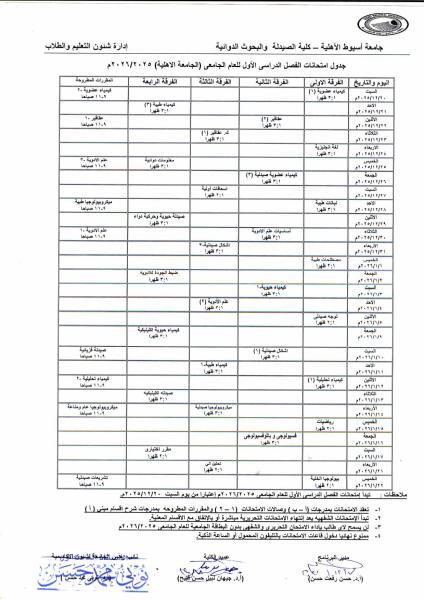
Examination Schedule for Students of Assiut University for the Humanities – Faculty of Pharmacy and Pharmaceutical Sciences First Semester, Academic Year 2025/2026
Meeting of the Libraries Committee of the Faculty of Pharmacy on Monday, 15 December 2025 at 12:00 PM

God willing, the meeting of the Libraries Committee at the Faculty of Pharmacy Pharmacy Pharmacy on Monday, 15 December 2025 at 12:00 PMat the invitation of Professor Mrs. Professor Dr. Gihan Nabil Hassan Fetih
This meeting will be held in the office of Prof. Dr. / Dean of the Faculty - Fifth Floor (Administrative Building).
The Faculty of Pharmacy council monthly meeting on Monday, 15 December 2025

God willing, the Faculty of Pharmacy council will hold its monthly meeting on Monday, 15 December 2025 at 11:00 (AM)
in the Hall of Faculty Council, 5th floor, administrative building
Meeting of the Executive Committee (Clinical Pharmacy Program) That will be on Monday, 15 December 2025

God willing, the Executive Committee meeting and That will be on Monday, 15 December 2025 at 10:30 AM. The meeting will take place in the Faculty Council Hall, 5th Floor (Administrative Building)