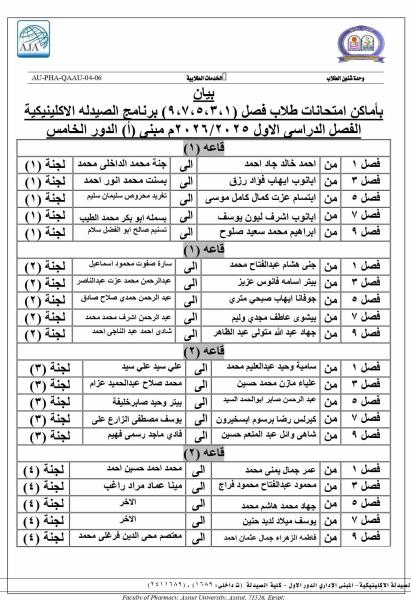
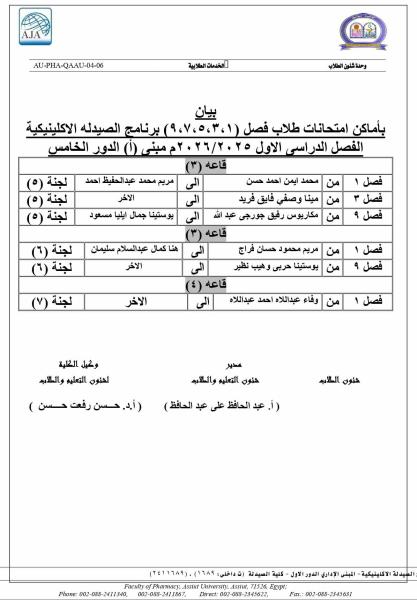
Do you have any questions? (088) 2080369 - 2345622 Pharmacy_QAAU@pharm.aun.edu.eg
Do you have any questions? (088) 2080369 - 2345622 Pharmacy_QAAU@pharm.aun.edu.eg
Ultrasensitive detection of zineb using a CoMn-MOF/rGO molecularly imprinted electrochemical sensor
Regulatory agencies have identified zineb (ZNB) as a potential health hazard due to its toxicological profile and environmental persistence. Therefore, establishing a highly selective and ultrasensitive method for ZNB detection is crucial for environmental monitoring, food safety assurance, and effective pesticide regulation enforcement. Herein, a selective electrochemical sensor was engineered based on a molecularly-imprinted polymer (MIP) film designed for targeted analyte recognition. The sensing platform integrates bimetallic cobalt–manganese metal–organic frameworks (CoMn-MOFs) with reduced graphene oxide (rGO) to enhance conductivity and surface activity. Initially, GO was synthesized and subsequently reduced to conductive rGO utilizing sodium borohydride via a modified Hummers’ method, forming a high-conductivity matrix for efficient electron transfer. Second, CoMn-MOFs were incorporated to significantly enhance the active surface area and facilitate electron transfer. A selective MIP layer was formed on the electrode surface via electro-polymerization, enabling precise molecular recognition of ZNB. The resulting MIP/rGO/CoMn-MOFs-modified glassy carbon electrode (GCE) exhibited excellent analytical performance, including a broad linear range (0.01–200 nM), a low LOD (4.0 pM), and high selectivity against potential interferents. When applied to real food and water samples, the sensor achieved high accuracy, with recoveries ranging from 95.5% to 105.6% and RSDs between 1.87% and 4.00%. The method was validated using the standard addition technique, confirming its applicability for accurate ZNB quantification in complex food and water matrices. These findings validate the sensor’s potential as a practical, rapid, and environmentally friendly platform for monitoring ZNB residues in agricultural and environmental contexts.
Integrating catalytic and fluorescence quenching strategies: A nanozyme-based platform for rutin sensing in complex matrices
Rutin is a potent antioxidant with therapeutic value in managing vascular and inflammatory conditions. Its accurate quantification is critical for pharmaceutical quality control and food safety. In this study, rutin was employed as a template to construct surface molecularly imprinted magnetic nanozymes (MIPs@Fe3O4–CoNi). These nanozymes retained peroxidase-like activity, catalyzing the H2O2-driven transformation of non-fluorescent terephthalic acid into fluorescent 2-hydroxyterephthalic acid. Upon rutin binding, catalytic activity was suppressed, and the fluorescence signal was further quenched through static and inner-filter effects. This dual-signal suppression mechanism significantly enhanced sensor sensitivity. The developed sensing platform exhibited a low detection limit of 0.008 μM and a broad linear range. When applied to pharmaceutical tablets, human serum, and food samples, it achieved recovery rates between 97.5 % and 104.0 %, with RSDs of 2.57 %–4.00 %. These results confirm the method's reliability, precision, and practical applicability across diverse matrices.
Switchable nanozyme-based colorimetric assay for protamine and heparin using silver citrate nanoparticles
Dual-mode nanozyme sensor based on molybdenum, boron, nitrogen, and sulfur co-doped carbon dots for sensitive determination of cefoperazone in milk, serum, and pharmaceutical injections
Monitoring cefoperazone (CFZ) in milk, serum, and pharmaceutical injections is essential for ensuring consumer safety, proper therapeutic exposure, and product quality. Residue control in milk protects against antimicrobial risks, while serum monitoring prevents toxicity or underdosing. Quality assessment of injections ensures formulation integrity throughout production. In this study, molybdenum–boron/sulfur co-doped carbon dots (Mo/B, N, S@CDs) were synthesized as a fluorescent nanozyme with strong peroxidase-like activity and a photoluminescence quantum yield of 43.55 %. In the presence of H₂O₂, the nanozyme catalyzed the oxidative coupling of 4-aminoantipyrine (4-AAP) with CFZ, producing a red quinoneimine dye with an absorption peak at 515 nm. This enabled colorimetric detection, with absorbance increasing proportionally to CFZ concentration. Concurrently, the dye quenched the green fluorescence of Mo/B, N, S@CDs at 530 nm, providing a sensitive fluorometric mode. The exceptional performance stems from synergistic Mo/B, N, S co-doping, which enhances peroxidase-like activity, optimizes electron transfer, and enables dual-mode signal amplification. Under optimized conditions, the colorimetric and fluorometric methods offered linear ranges of 0.1–500 μM and 0.01–500 μM with detection limits of 0.018 μM and 0.008 μM, respectively. The dual-mode platform performed reliably in milk, serum, and injection samples, yielding recoveries of 98.0–105.7 % and RSDs below 4.00 %, demonstrating its suitability for routine CFZ monitoring.
Dual-mode colorimetric and fluorometric detection of D-penicillamine via inhibition of peroxidase-mimetic activity of bimetallic N-doped carbon dots
D-penicillamine (D-PEN) is used to treat Wilson's disease, rheumatoid arthritis, and cystinuria but requires careful monitoring due to its narrow therapeutic window and risks of nephrotoxicity, hematological disorders, and autoimmune reactions. To enable reliable detection, bimetallic FeCu co-doped carbon dots (FeCu@CDs) were developed as multifunctional nanozymes for dual-mode sensing of D-PEN. These nanozymes integrate peroxidase-like catalytic activity, intrinsic fluorescence, and selective thiol-binding affinity, allowing simultaneous colorimetric and ratiometric fluorescence detection. Fe and Cu doping enhanced the peroxidase-like activity and fluorescence emission of the CDs by promoting H₂O₂ activation into HO• and 1O₂ and improving charge mobility. D-PEN inhibited this catalytic activity by binding metal centers and altered the inner filter effect (IFE) between FeCu@CDs and 2, 3-diaminophenazine (DAP), enabling selective signal modulation. The assay achieved detection limits of 0.13 μM (colorimetric) and 0.03 μM (fluorescence), with recoveries ranging from 95.6 % to 105.1 % in real samples, highlighting its importance in clinical monitoring and bioanalytical applications.
Low-energy room-temperature carbon dots for targeted sensing of MET inhibitor capmatinib
Capmatinib (CMB) monitoring in biological fluids is critical for evaluating its pharmacokinetics, optimizing
dosing, and minimizing toxicity. Accurate measurement is essential for ensuring therapeutic efficacy,
enabling personalized treatment, and preventing adverse effects. Given the variability in patient
metabolism and excretion, regular monitoring helps maintain CMB levels within the therapeutic range,
improving treatment outcomes and minimizing the risk of drug resistance. This work presents an
economical and energy-efficient strategy for preparing highly luminescent nitrogen-doped carbon dots
(NCDs), employing 2,5-dihydroxy-1,4-benzoquinone alongside triethylenetetramine. The synthesized
NCDs demonstrated excellent photostability and a high fluorescence quantum yield of 38.72%. Upon the
addition of CMB, concentration-dependent fluorescence quenching was observed at 515 nm, which was
attributed to the inner filter effect (IFE), with LOD of 3.6 nM. The NCDs exhibited high selectivity in
detecting CMB, with minimal cross-reactivity from simultaneously present compounds. Recovery studies
in real biological samples yielded rates between 97.4% and 105.3%, and RSDs were consistently below
4.11%. These results demonstrate the method's precision, reproducibility, and potential for reliable CMB
detection in complex biological matrices.
Repurposing disposable medical syringes into valuable fluorescent carbon dots: application to the fluorometric determination of nintedanib
Monitoring nintedanib (NTB) using reliable analytical methods is essential for ensuring safe dosing, minimizing toxicity, assessing drug–drug interactions, and supporting quality control in personalized cancer therapy. In this work, we present a cost-effective and energy-efficient hydrothermal strategy for synthesizing nitrogen-doped carbon dots (CDs) from disposable plastic syringes. The process, carried out at 200 °C following a calcination pretreatment, not only provides a sustainable route for valorizing biomedical waste but also addresses pressing environmental challenges. The as-prepared CDs exhibited intense green fluorescence, outstanding photostability, and a high quantum yield of 46.42%, reflecting their superior optical performance. Upon exposure to NTB, a concentration-dependent quenching response at 470 nm was observed, primarily mediated by the inner filter effect (IFE). This mechanism enabled highly sensitive NTB detection, with an ultralow detection limit of 2.5 nM (S/N = 3). The probe demonstrated remarkable selectivity, showing negligible interference from common coexisting ions, biomolecules, and anticancer drugs. Analytical accuracy was validated by recovery studies in spiked serum and urine samples, which ranged from 97.6% to 103.2%, while RSD values below 3.48% confirmed excellent precision and reproducibility. These findings establish the proposed CD-based probe as a robust, reproducible, and clinically relevant tool for NTB quantification. By demonstrating the conversion of discarded medical plastics into high-value nanomaterials, this work presents a strategy that aligns with the goals of green nanotechnology and delivers a practical platform for bioanalytical sensing, therapeutic drug monitoring, and pharmacokinetic studies. The dual focus on waste repurposing and clinical utility underscores the potential the potential of syringe plastic-derived CDs for translation into next-generation biomedical diagnostics.
Selective detection of tyrosine kinase inhibitor erdafitinib using nitrogen-doped carbon dots synthesized at room temperature
Erdafitinib (ERDF), despite its known adverse effects, remains a key chemotherapeutic agent for metastatic urothelial carcinoma, requiring reliable monitoring in biological matrices to ensure safe and effective treatment. In this study, we developed a cost-effective, energy-efficient method for synthesizing highly fluorescent nitrogen-doped carbon dots (NCDs) using simple precursors. The resulting NCDs displayed excellent photostability and a high fluorescence quantum yield of 41.87%. ERDF induced a concentration-dependent quenching of the NCDs' fluorescence at 520 nm, attributed to the inner filter effect, with a low detection limit of 0.013 μM (S/N = 3). The NCDs demonstrated high selectivity toward ERDF with minimal interference from coexisting substances. Recovery rates in spiked human serum and urine ranged from 97.8% to 104.4%, validating the method's practical applicability. The relative standard deviations (RSDs) remained below 3.72%, confirming the precision and reproducibility of the NCD-based sensing platform.
Nickel ferrite–halloysite nanocomposite-modified GCE for nanomolar detection of purine bases: Toward early disease diagnosis
Purine bases such as adenine and guanine are fundamental components of nucleic acids and are involved in critical metabolic processes. Abnormal elevations in their concentrations have been associated with various pathological conditions, including cancer. In this study, a highly sensitive voltammetric sensor was developed by modifying a glassy carbon electrode (GCE) with nickel ferrite nanoparticles (NiFe₂O₄ NPs) anchored onto halloysite nanotubes (HNTs), forming a NiFe₂O₄ NPs@HNTs composite. The synergistic integration of redox-active NiFe₂O₄ NPs and high-surface-area, biocompatible HNTs significantly enhanced the electrochemical response by increasing the active surface area, improving electron transfer kinetics, and facilitating strong analyte adsorption. This composite enabled well-defined, irreversible oxidation peaks for both adenine and guanine, with a wide linear detection range from 0.05 to 200 μM. The calculated limits of detection (LODs) were 2.2 nM for adenine and 1.4 nM for guanine, indicating excellent sensitivity. When tested in pre-treated human saliva and serum samples, the sensor achieved recovery rates ranging from 95.7% to 105.2%, with relative standard deviations below 4.21%, confirming its accuracy and reliability in complex biological matrices. These findings highlight the sensor’s strong potential for future clinical applications in purine base monitoring and biomarker-based disease diagnostics.